Decree no. 2010-49 of January 13, 2010 on medical biology (LBM), ratified by Law no. 2013-442 of May 30, 2013 on the reform of medical biology, requires the accreditation of all activities of medical biology laboratories (LBM). This accreditation is issued by the French Accreditation Committee (COFRAC) and is based on the international NF EN ISO 15189 standard supplemented by the NF EN ISO 22870 standard (delocalized biology) for medical biology activities.
Web Access to regulatory standards
Accreditation in Henri Mondor hospital
Accreditation is the recognition by a third-party body of the competence of a body in a specific domain. It is proof that the laboratory meets certain requirements concerning technical skills and system management, which are necessary to continuously obtain valid results.
The goal of seeking accreditation is to establish confidence in the services provided.
Accreditation is delivered by COFRAC for a period of four years for the first cycle and subsequently renewed for a maximum period of five years.
For several years now, the Laboratory of Virology, at the Henri Mondor Hospital has been committed to seeking accreditation. The laboratory was audited by COFRAC in February 2014 and then again in May 2015 for activities related to infectious diseases. The audit in 2014 granted the accreditation of the Biology and Pathology Division at the Henri Mondor Hospital (under number 8-3372).
In 2016, the Biology and Pathology Division was accredited, for approximately 60% of its total activity. The objective is to meet regulation requirements and acquire accreditation for 100% of its activities by 2020.
The Quality management system set up by the Biology and Pathology Division is based on the following 10 processes: quality, service contracts and agreements, pre-analytic, analytic, post-analytic, human resources, the laboratory computer system, management of equipment, reagents and consumables, metrology, and hygiene, security, and the environment.
The quality management system applies to the entire activity of the lab and consists of:
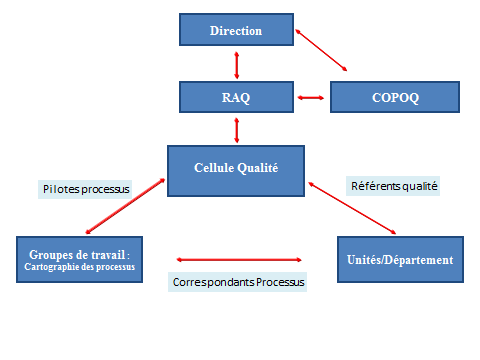
Document control is based on a document management procedure. The purpose of this procedure is to describe the measures used to ensure the control of internal and external documentation in the Biology and Pathology Division, from the expression of the need of a document to its destruction or archiving.
The Biology and Pathology Division records and considers client complaints (clinical units, patients, other medical biology laboratories, and the Infection Control team). Two options exist for submitting a complaint:
A quality assurance manual is available to clients of the Biology and Pathology Division.
A sample manual is available on the intranet. It includes documents related to the division, its description, its requirements, and, in particular, those of the Laboratory of Virology, as well as an examination guide outlining the conditions for taking, storing, and transporting samples, etc.
As part of its activities, the Laboratory of Virology is responsible for carrying out examinations from other AP-HP hospitals, public or private facilities, and medical biology laboratories.
Signed agreements between the management of the University Hospital Henri Mondor and stakeholders allow the verification of respective requirements, together with a review of the laboratory’s capacity to carry out examination requests.